1. Energy: Conceptual Foundations and Governing Transformations
Recognizing various forms of energy and understanding the scientific principles involved in the transformations that take place between these energy categories is fundamental to scientific understanding at any level of inquiry. Energy is found in the form of molecular motion, electrical energy, nuclear energy, electromagnetic energy, kinetic energy of macroscopic objects, gravitational energy, etc. The scientific principles that govern energy at the molecular level are linked to the behavior of energy at the global level. This establishes a foundation defining the central importance of energy for each of the subsequent chapters. | 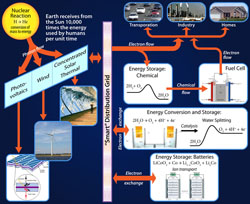 |
2. Atomic and Molecular Structure: Energy From Chemical Bonds
How is the energy contained in the chemical bond extracted to produce useful work at the molecular level? Why is virtually all of the energy contained in a chemical bond lost as heat under some circumstances, but is effectively channeled to build new and complex molecules in other instances? How is the chemical energy of one reaction coupled to subsequent chemical reactions leading to the formation of a desired chemical product? These questions are explored by first developing the concepts central to atomic structure and chemical bonding. | 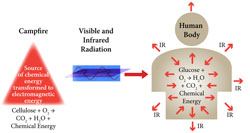 |
| 3. Thermochemistry: Development of the First Law of Thermodynamics The First Law of Thermodynamics is the law of conservation of energy: Heat and work are both forms of energy. In any process, energy can be converted from one form to another, but it is never created nor destroyed. In order to understand and apply the First Law, we must develop a clear understanding of the distinction between temperature, heat, work and the thermal energy contained within the system under study. These ideas are developed quantitatively such that they can be applied to chemical reactions, heat engines and phase changes. | 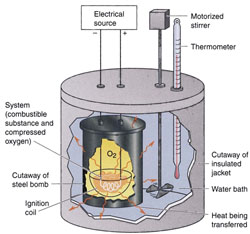 |
4.Entropy, Free Energy and the Second Law of Thermodynamics
Nature is driven by spontaneous processes, processes that proceed without external intervention. With our advancing understanding of energy, with the insight brought by the First Law of Thermodynamics, and with our ability to track energy transformations from highly organized forms of energy that inexorably cascade to disorganized thermal energy, comes a sharpened recognition that there are other factors that drive spontaneous processes in nature. This constitutes the imperative for understanding entropy. |  |
5. Equilibria and Free Energy
A chemical system in equilibrium, aA + bB <=> cC + dD, represents an unchanging combination of macroscopic properties: concentrations, pressure, temperature, etc. There are no apparent changes with time. The equilibrium state thus determines the extent to which a reaction takes place. But at the molecular level, the situation is far different. In this section we quantitatively couple the concept of Gibbs Free Energy with the concept of chemical equilibrium. | 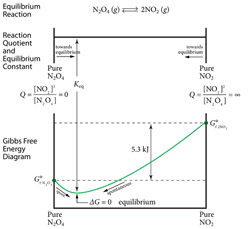 |
6. Acid-Base Control of Life Systems
The role of proton transfer—the transfer of a hydrogen ion, H+, from one species to another, often involving water as a solvent—is a theme of broad importance. The proton, as we will see, organizes the molecular level structure of liquid water, controls life’s biochemical pathways, is held in delicate control in the blood of all organisms, constructs and deconstructs polymers, and aids in the synthesis of exquisite calcium containing structures at the intersection of organic and inorganic architectures in living systems. This section links the behavior of acids and bases to Gibbs Free Energy. |  |
7. Electrochemistry
The study of the union between chemistry and electricity—the flow of electrons driven spontaneously by Free Energy release in a chemical reaction—has provided a rich history of remarkable discoveries. These studies constitute the discipline of electrochemistry, a subject that has experienced a dramatic rebirth. That rebirth has been propelled by the emergence of energy production and storage as a dominant problem confronting both science and public policy. New developments in electric automobiles are linked to important developemnts to control the release of carbon to the atmosphere. |  |
8. Quantum Mechanics, Wave-Particle Duality and the Single Electron Atom
Development of the quantum theory of atomic structure begins with a treatment of the distinction between properties associated with waves and the properties associated with particles. With the hypothesis put forward by the French physicist Louis de Broglie that the electron was in fact a “matter wave” with wavelength λ = h/p came the advent of quantum mechanics and the birth of modern chemical physics. Quantum mechanics recognized that at the atomic scale, electrons were controlled by their wave properties, and those wave properties were in turn defined mathematically by the wavefunction, ψ, of the electron. | 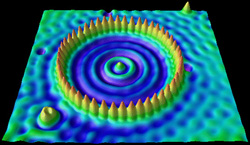 |
9. Quantum Mechanics of Multi-Electron Systems
Quantum mechanics introduced three key attributes of the behavior of electrons at the atomic scale: (1) the wave properties of the electron when confined to the spatial scale of atoms and molecules, (2) the electron spin that dictates the number of electrons allowed in any atomic (or molecular) orbital, and (3) the placement of electrons in, and the transfer of electrons between, both occupied and unoccupied energy levels. It is quantum mechanics that continues to bring a revolution in our understanding that has opened a gateway to new materials with properties that were unimagined just a few years ago. |  |
10. Theories of Molecular Bonding I: Valence and Shared Electron Structures
In this section we develop the theoretical basis for the molecular bond that provides a means for understanding both the structure of the molecular bond and the electron assignments in the Lewis structure of bonding theory. We develop: the structure of the molecular bond, types of chemical bonds that result from different categories of relative electronegativity in bonds, Lewis structure for ionic bonds, Lewis structure for diatomic and polyatomic molecules, and determination of molecular shapes (valence shell electron pair repulsion theory, VSEPR). | 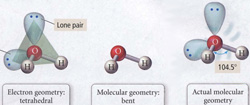 |
11. Theories of Molecular Bonding II: Quantum Mechanically Based Theories
The quantum mechanical formulation of covalent bonding constitutes a cornerstone of modern chemistry. In this section we develop molecular bonding in the context of quantum mechanics using two complementary approaches. First we develop the Valence bond (VB) formulation of molecular bonding that combines atomic orbitals to form bonding structures with the electrons assigned to those atomic orbitals. Second, we develop the molecular orbital (MO) theory of chemical bonding that takes linear combinations of atomic orbitals to form new molecular orbitals that are distributed such as to reduce the potential energy of the molecular structure. | 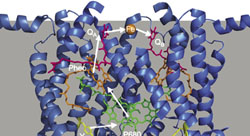 |
12. Kinetics and Photochemistry
Chemical kinetics is a study of the rate at which chemical transformations take place, and it is a subject central to modern chemistry in a number of key areas. In particular kinetics: defines key pathways in organic synthesis, polymers, materials and drug development, constitutes the key to determining metabolic rates in living organisms, controls pollutants from energy sources and is thus central to human health, is key to developments of nano-electronics and computer memory fabrication, establishes the foundation of forensic medicine, and is a critical diagnostic for restructuring world energy strategy. | 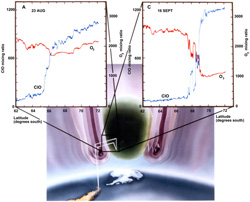 |
13. Structure, Properties and Technology of Materials
Quantum mechanics establishes the foundation for understanding the properties of all materials, but in particular an understanding of molecular bonding provides the basis for understanding the electrical characteristics of materials. This in turn establishes how semiconductors are created and how the combination of different semiconductors provide the underpinning for an array of modern electronics from transistors, to microchips, to computers, to flat-screen televisions. In addition the interaction of photons with molecular structures opens the path for the generation of energy from sunlight. |  |
14. Nuclear Chemistry
Nuclear chemistry addresses key questions engaging energy generation, nuclear waste, and arms control related to national security. Nuclear chemistry also has at the foundation of medical imaging, radiocarbon dating of archaeological findings and isotopic analysis associated with climate studies, mineral extraction and cosmology. |  |